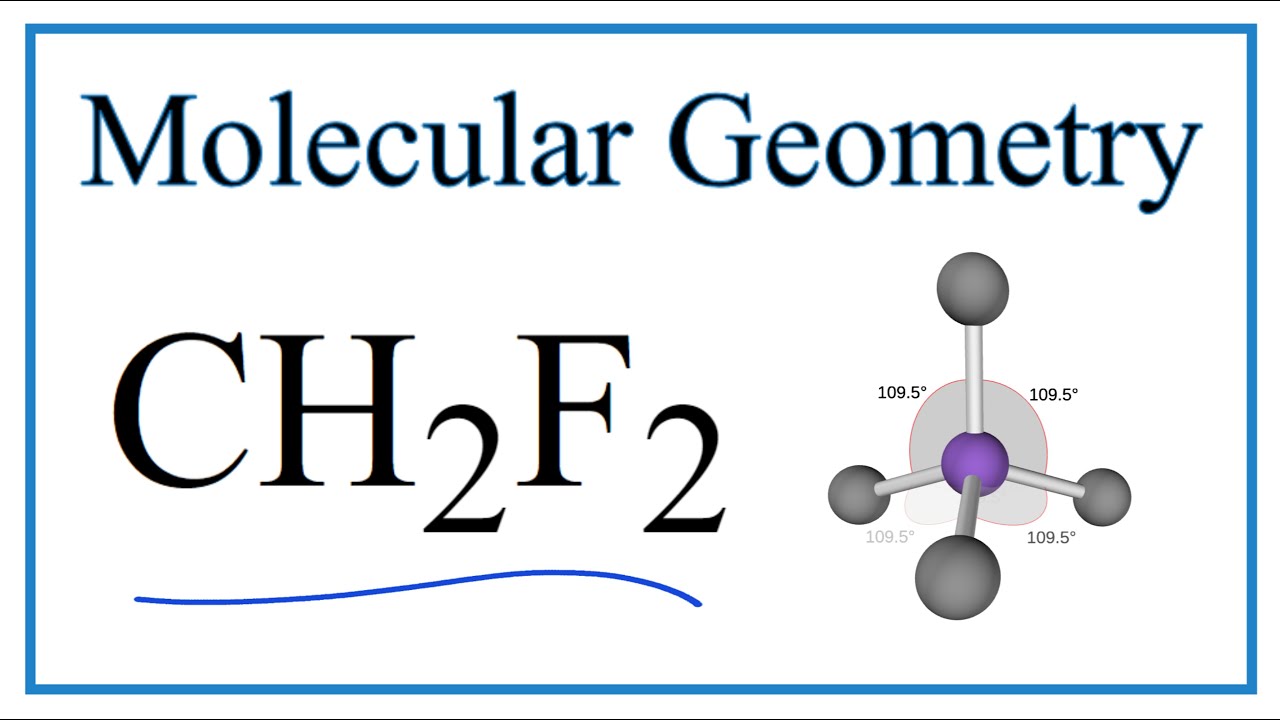
The molecular geometry of CH2F2, also known as dichloromethane or DCM, plays a crucial role in its physical and chemical properties. Understanding the arrangement of atoms in a molecule is essential for predicting its behavior and interactions with other substances. In this article, we will discuss the molecular geometry of CH2F2 and its implications. To begin, lets briefly review the structure of CH2F2. It consists of one carbon atom (C) bonded to two hydrogen atoms (H) and two fluorine atoms (F). The carbon atom is located at the center of the molecule, with the hydrogen and fluorine atoms arranged around it. The molecular formula for CH2F2 is CH2F2, indicating that it contains two hydrogen atoms, one carbon atom, and two fluorine atoms. To determine the molecular geometry of CH2F2, we need to consider the arrangement of atoms and the presence of lone pairs. In this case, the carbon atom forms four bonds, two with hydrogen and two with fluorine. To minimize electron repulsion and achieve maximum stability, the atoms in CH2F2 adopt a tetrahedral arrangement. The tetrahedral molecular geometry of CH2F2 can be visualized as a carbon atom at the center, with four sp3 hybrid orbitals pointing towards the corners of a tetrahedron. Two of these sp3 orbitals form sigma bonds with hydrogen atoms, and the other two form sigma bonds with fluorine atoms. The lone pairs of electrons on the fluorine atoms are not considered in the molecular geometry, as they do not significantly affect the overall shape of the molecule. The tetrahedral molecular geometry of CH2F2 has several implications for its physical and chemical properties. First, the symmetry of the molecule leads to a nonpolar nature. Since the carbon-hydrogen and carbon-fluorine bonds have similar electronegativities, the electron distribution in the molecule is relatively symmetrical. As a result, CH2F2 does not have a net dipole moment, making it nonpolar. The nonpolar nature of CH2F2 influences its solubility in different solvents. Being nonpolar, CH2F2 is soluble in nonpolar solvents like benzene, hexane, and chloroform. On the other hand, it is not soluble in polar solvents like water, which favors interactions with other polar molecules. Another important aspect of the molecular geometry of CH2F2 is its bond angles. In a tetrahedral arrangement, the bond angles between the carbon-hydrogen and carbon-fluorine bonds are approximately 109.5 degrees. These bond angles are determined by the repulsion between electron pairs around the central carbon atom. The relatively large bond angles in CH2F2 contribute to its unique physical properties. The tetrahedral molecular geometry of CH2F2 also affects its reactivity and chemical behavior. The carbon-fluorine bonds in CH2F2 are relatively strong due to the high electronegativity of fluorine and the overlap of sp3 hybrid orbitals. As a result, CH2F2 is resistant to nucleophilic substitution reactions, where a nucleophile replaces a leaving group attached to the carbon atom. However, it can undergo reactions with strong bases or oxidizing agents under certain conditions. In conclusion, the molecular geometry of CH2F2 is tetrahedral, with a carbon atom at the center bonded to two hydrogen atoms and two fluorine atoms. The tetrahedral arrangement is determined by the need to minimize electron repulsion and achieve maximum stability. The nonpolar nature of CH2F2, resulting from its symmetrical electron distribution, affects its solubility and interactions with other molecules. The bond angles in CH2F2, around 109.5 degrees, contribute to its physical properties. Understanding the molecular geometry of CH2F2 is essential for comprehending its behavior and reactivity in various chemical reactions.
CH2F2 Molecular Geometry, Bond Angles (and Electron Geometry). CH2F2 Molecular Geometry, Bond Angles (and Electron Geometry) - YouTube An explanation of the molecular geometry for the CH2F2 (Difluromethane) including a description of the.sweepstakes promo text message
. CH2F2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity .. The molar mass of difluoromethane is 52.024 g/mol molecular geometry of ch2f2
hentai where guy has a pass to fuck anyone to find a speciap one
. CH2F2 contains fluorine, an electronegative element, but still, it does not show hydrogen bonding owing to the absence of an H-F bond. molecular geometry of ch2f2. Difluoromethane - Wikipedia molecular geometry of ch2f2. It has the formula of CH 2 F 2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stabilityyou need to back the fuck up. you"re getting too close. blaha
. [2] [failed verification] Due to the low melting and boiling point, (-136.0 °C and -51.6 °C respectively) contact with this compound may result in frostbite.. CH2F2 Lewis structure, Hybridization, Molecular . - Geometry of Molecules. The chemical formula CH2F2 represents Difluoromethane molecular geometry of ch2f2. Being a refrigerant, Difluoromethane is also known as HFC-32 or, more commonly, R-32 molecular geometry of ch2f2. Here, two Fluorine atoms take the place of Hydrogen atoms in Methane (CH4) to form CH2F2. It is a colorless and odorless gas that is also mildly inflammable.. CH2F2 Molecular Geometry, Bond Angles & Electron Geometry . - YouTube

where can i find girls that want to fuck
. In this article, we will discuss CH 2 F 2 lewis structure, molecular geometry or shape, bond angle, polar or nonpolar, its hybridization, etc. It has high thermal stability.. Solved CH2F2 • Draw the lewis dot structure Determine the | Chegg.com. Question: CH2F2 • Draw the lewis dot structure Determine the molecular geometry and draw its accurately Indicate the polarity of any polar bonds within the structure Classify the molecule as polar or nonpolar • Clearly explain results, and explain the reasoning used to determine the answers | molecular geometry of ch2f2workers who lead collective bargaining efforts crossword
. CH2F2 - University of Wisconsin-Oshkosh. Table 1: Bond lengths from the literature 2 molecular geometry of ch2f2. Note that for C-H and C-F both bonds are the same length. Table 2: Bond angles from literature 2best sex dating sites 2016 free app

i just want to fuck but no onenis available
. The single pairs of atoms oppose each other, thus forcing the other atoms to move away. VSEPR Theory It is believed that the VSEPR Theory is a model which can assist in predicting the structure of molecules. molecular geometry of ch2f2. CH2F2 Lewis structure - Learnool. In the CH 2 F 2 Lewis structure, there are four single bonds around the carbon atom, with two hydrogen atoms and two fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. Steps. #1 Draw a rough skeleton structure
2010 ford fusion belt diagram 4 cylinder
. #3 If needed, mention formal charges on the atoms.. Difluoroacetylene | C2F2 | CID 136491 - PubChem. PubChem 2 Names and Identifiers 2.1 Computed Descriptors 2.1.1 IUPAC Name 1,2-difluoroethyne Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) PubChem 2.1.2 InChI InChI=1S/C2F2/c3-1-2-4 Computed by InChI 1.0.6 (PubChem release 2021.05.07). How to draw CH2F2 Lewis Structure? - Science Education and Tutorials. The molecule of difluoromethane (with tetrahedral molecular geometry) is tilted, the bond angles between fluorine, carbon, and hydrogen (H-C-H and F-C-F) are 112.5 and 108.5 degrees. It has a difference in electronegativity values between carbon and fluorine atoms, with carbons pull being less than fluorines terminal in the CH2F2 molecule.. Lewis Structure of CH2F2 - Root Memory. By using the following steps, you can easily draw the lewis structure of CH 2 F 2. Step #1: draw skeleton. Step #2: show chemical bond